The study of ancient DNA has undergone nothing short of a revolution in the past two decades. What began as tentative attempts to extract genetic material from long-dead organisms has blossomed into a field that is fundamentally rewriting our understanding of human history, evolution, and even modern medicine. From revealing our intimate connections with Neanderthals to tracing the origins of devastating diseases, ancient genomics has become one of the most transformative scientific disciplines of our time.
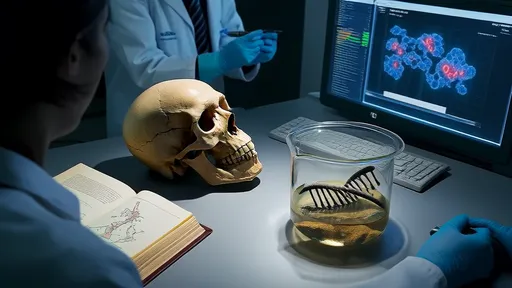
The accidental discovery that changed everything came in 1997 when researchers managed to sequence mitochondrial DNA from the original Neanderthal type specimen found in Germany's Neander Valley. This first glimpse into our extinct cousins' genetics suggested they were indeed a separate lineage from modern humans. But the real bombshell would come more than a decade later with the first draft sequence of the complete Neanderthal genome.
When Svante Pääbo and his team at the Max Planck Institute published the Neanderthal genome in 2010, they made an astonishing discovery - modern humans outside Africa carry between 1-4% Neanderthal DNA. This proved that our ancestors interbred with Neanderthals after migrating out of Africa, overturning the previous consensus that Homo sapiens simply replaced other hominins without mixing. The implications were profound: we hadn't just coexisted with our evolutionary cousins, we'd had children with them.
Subsequent research has revealed even more complex interactions between different groups of ancient humans. Denisovans, another extinct hominin identified solely through DNA from a finger bone found in Siberia, left their genetic mark on modern populations in Asia and Oceania. Some groups carry Denisovan DNA that appears to confer advantages for living at high altitudes. Even more remarkably, traces of "ghost populations" - groups we know only from their genetic legacy in modern humans - continue to be discovered through sophisticated analysis of ancient and contemporary genomes.
The ancient DNA revolution hasn't just illuminated human evolution - it's provided startling insights into the history of diseases that still affect us today. In 2011, researchers sequenced the genome of the bacterium that caused the Black Death from victims buried in London's East Smithfield plague pits. This confirmed that Yersinia pestis was indeed the culprit behind history's most devastating pandemic. More recently, studies of ancient tuberculosis DNA have traced the disease's co-evolution with humans back over 6,000 years.
Perhaps most remarkably, ancient DNA is helping explain why some populations are more vulnerable to certain modern diseases than others. The same Neanderthal DNA that helped our ancestors adapt to new environments outside Africa may contribute to autoimmune disorders, depression, and addiction susceptibility today. This evolutionary trade-off - where genetic variants that were once advantageous become detrimental in modern environments - is a major focus of current research.
The technological advances driving this field have been breathtaking. Early ancient DNA studies could only analyze short mitochondrial sequences, but next-generation sequencing now allows researchers to reconstruct entire genomes from incredibly degraded material. New techniques to distinguish ancient DNA from modern contamination have been crucial, as have methods to extract genetic material from increasingly challenging sources - whether it's 7,000-year-old earwax or 2-million-year-old environmental DNA preserved in permafrost.
Ethical considerations have grown alongside these technical capabilities. Many indigenous communities have legitimate concerns about the study of ancestral remains, leading to important discussions about consent, ownership, and appropriate handling of ancient DNA. Some Native American groups have successfully pushed for the repatriation and reburial of remains that were previously subject to genetic analysis without community consultation. These conversations are reshaping how ancient DNA research is conducted worldwide.
The medical potential of ancient DNA research is only beginning to be realized. By understanding how ancient humans adapted to pathogens, we may develop new approaches to combat antibiotic resistance. The study of archaic immune genes could inspire novel therapies for modern diseases. Even the peculiar biology of extinct hominins holds promise - Neanderthal variants of certain genes are being studied for their potential effects on pain tolerance and blood clotting.
Looking ahead, the field faces both exciting opportunities and significant challenges. Pushing the boundaries of how far back we can sequence DNA could reveal entirely unknown branches of the human family tree. Applying ancient DNA techniques to more recent historical periods promises to rewrite our understanding of major events like the Anglo-Saxon migration to Britain or the peopling of the Americas. However, the destructive nature of sampling ancient remains means researchers must carefully balance scientific discovery with preservation of irreplaceable artifacts.
What began as a niche field at the fringes of genetics has become central to our understanding of what makes us human. The ancient DNA revolution has shown that our species' history is far more complex and intertwined than we ever imagined. As techniques improve and more genomes are sequenced, we can expect even more startling revelations about our shared past - and how it continues to shape our present.

By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025
By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025